Every week, Madison (my 10-year old daughter) and I teach a 1.5-hour afterschool science club for K-2nd graders. Each week she helps plan the lesson and then we write the blog about what we did.
We learned most of the science experiments and activities from Steve Spangler (awesome speaker and science guy extraordinaire). We have included links to his science supplies, experiments, and videos. We also include links to our YouTube videos and other products that we used. These links take you away from the blog and to external websites.
Lesson 5
Science of Air Pressure II
Science Standards Addressed
- Observe that things (air) move in different ways.
- Describe the characteristics of the 3 states of matter.
- Know that air takes up space and exerts a force.
Plus we snuck in some speaking and listening standards like asking questions, expressing ideas, following multi-step directions, and participating in discussions.
10 minutes
As the children arrived we played the songs In the Air Tonight, by Phil Collins, and The Air that I Breathe, by the Hollies.
Next we had a snack that once again was a hint of the science to follow.
 |
| In addition to the healthy stuff, we snacked on marshmallows (full of air) and AIR Heads! |
To prime their brains, as they drank their juice (with straws) we taught them that they weren’t actually sucking juice into their mouths.
 |
| They were decreasing the air pressure in their mouths (partial vacuum), so that the atmospheric pressure could push the juice up the straw and into their mouths. |
While we ate, Madison read
 |
| Curious George and the Hot Air Balloon by Margret and H.A. Rey |
a book about George the monkey who, because of curiosity, accidently unhooked a hot-air balloon and sailed away. He flew past George Washington’s nose on Mt. Rushmore. In the end he saved a stranded worker; George was a hero!
It also contains an accurate depiction of how they use Bernoulli's Principle to inflate a hot-air balloon.
10 Minutes
Mysterious Water Suspension To really engage the kids, we started with a really exciting demo. We asked them to watch carefully and to predict what might happen next. We filled a glass jar to the rim with water. Then we placed the card over the rim like a lid. Keeping pressure on the card, we turned the jar upside down and asked the kids what they thought might happen if we released the pressure we were applying to the card and removed our hand. Some of the kids predicted that the water and card would fall if we removed our hand.
Mysterious Water Suspension To really engage the kids, we started with a really exciting demo. We asked them to watch carefully and to predict what might happen next. We filled a glass jar to the rim with water. Then we placed the card over the rim like a lid. Keeping pressure on the card, we turned the jar upside down and asked the kids what they thought might happen if we released the pressure we were applying to the card and removed our hand. Some of the kids predicted that the water and card would fall if we removed our hand.
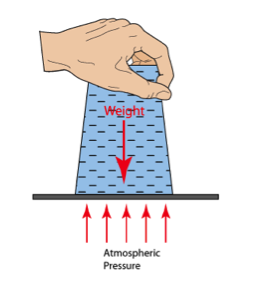 |
| Some of them had already seen this demo (or so they thought) and predicted that the card would remain in place because of the atmospheric pressure pushing up. |
We asked for a BRAVE volunteer that wasn’t afraid to get soaked! Everyone volunteered. We picked one and asked him to come stand under the upturned jar. I removed my hand and he did a little “I told you I wouldn’t get wet” dance. We asked him to look up at the card and read what it said. He read it aloud for the group, “DO NOT REMOVE THIS CARD.” We asked him to predict what would happen if we removed the card. Everyone agreed that he would get soaked. We asked him if he was STILL feeling brave. When he agreed to stay, we removed the card! A little bit of water leaked out, but the rest of the water remained in the jar!
How could this be? The secret is a little circle of window screen spanning the jar opening. It’s all about water molecules and how they cling to each other forming "surface tension" – just enough cling, combined with atmospheric pressure to keep the water above the screen.
10 Minutes
You’ve Got Me Under Pressure To get the kids excited and to demonstrate atmospheric pressure; we brought out an old classic - the pressure mat.
You’ve Got Me Under Pressure To get the kids excited and to demonstrate atmospheric pressure; we brought out an old classic - the pressure mat.
 |
| You just place this rubber mat on a clean flat surface and ask a volunteer to lift the mat by the handle – straight up – no sliding it off the edge! |
It is almost impossible, but YOU can pick it up easily (by the edge – be sneaky).
The science? Ever heard the expression “light as air”?
 |
| Air isn’t light at all. The air surrounding our planet weighs a lot. |
 |
| This heavy “sea” of air exerts a LOT of pressure. The atmospheric pressure at sea level is 14.7 pounds per square inch of surface area. |
 |
| That’s roughly the weight of 2 gallons of milk resting on 1 square inch! So, the mat has about 1,620 pounds of atmospheric pressure pushing down on it! |
45 Minutes
Honey, I Shrunk the Marshmallows
Honey, I Shrunk the Marshmallows
For this experiment, we used a Fizz-Keeper. Fizz-Keepers are the little pressure pumps designed to keep the fizz in PLASTIC bottles of soda that have previously been opened.
 |
| We filled empty baby soda bottles and 16 oz. bottles about half full with marshmallows, and screwed on the pumps. |
As we began pumping, we quickly noticed how the marshmallows seemed to wrinkle and shrink as the pump forced molecules of air (that pushed on the marshmallows) into the bottle.
 |
| Since marshmallows are mostly pockets of air, the increased pressure compacts the air molecules and the marshmallows shrink. |
Then we released the pressure by unscrewing the pump. It was really cool to see the marshmallows poof back up. When we unscrewed the pump, the air pressure inside the marshmallow bubbles was greater than the pressure pushing on the marshmallow from the outside, so the marshmallows proofed back up.
Safety Notes: This requires adult supervision – preferably a responsible adult! Use only plastic soda bottles that are in good shape. Do not pump more than 40 strokes (pumps) into a 16 oz. bottle. Never leave a soda bottle in a pressurized state.
Apply only enough pressure to allow you to see the shrinking effects.
You think that was cool? We kicked it up a notch by reversing the process.
 |
| My dad bought a nice (expensive) a vacuum packing food saver on the way to school just for this experiment. My mom rolled her eyes. |
You can find cheaper models that will work just fine on the Internet. They are great for exploring the properties of air pressure.
 |
| We filled the storage container with marshmallows and removed a lot of the air. |
 |
| Honey, we blew up the marshmallows! Please note: No actual marshmallows were harmed during the making of this experiment. |
We brainstormed other experiments we could do with the food saver. We could use a small balloon or a bag of potato chips in place of the marshmallows. What if we put in soap bubbles, shaving cream, or silly string? If a Fizz Keeper keeps sodas fizzy, will the food saver make them flat?
20 Minutes
Shrink Wrapped Kids
Shrink Wrapped Kids
This also needs adult supervision – try to find a mature one.
Never EVER put a bag over anyone’s head (we said this over and over again, but the kids all knew never to do that)!
We asked parent and child volunteers to step into a BIG plastic bag. We asked them to cup their hand around a vacuum cleaner nozzle that we inserted into the bag (this keeps the bag from being sucked into the nozzle). We tried to create as good a seal as we could just above their shoulders.
 |
| We turned on the vacuum, and in a few seconds the unsuspecting victim… err, I mean volunteer gets shrink wrapped. It feels CRAZY! |
We snuck in a little social studies when we talked about how this science helps to package and preserve food by cryovac or shrink-wrap packaging.
Steve credits Wayne Goates with this demo – the guy who invented the shrink wrap your sweaters as seen on TV. Reference: Wayne Goates. NMLSTA Level Line. "Shrink Wrap With A Vacuum," Winter, 1996.








































